Publications
Selected Publications (Bucknell students underlined)
F Whiting‐Fawcett, AS Blomberg, T Troitsky, MB Meierhofer, KA Field, SJ Puechmaille, TM Lilley, A Palearctic view of a bat fungal disease, Conservation Biology 39:1, e14265 (2025) Open Access Full Text
EL Pannkuk, MS Moore, S Bansal, K Kumar, S Suman, D Howell, JA Kath, A Kurta, DM Reeder, KA Field, White adipose tissue remodeling in little brown myotis (Myotis lucifugus) with white-nose syndrome, Metabolomics 20:5, 100 (2024) doi:10.1007/s11306-024-02165-4
VG Twort, VN Laine, KA Field, F Whiting-Fawcett, F Ito, M Reiman, T Bartonicka, M Fritze, VA Ilyukha, VV Belkin, EA Khizhkin, DM Reeder, D Fukui, TL Jiang, TM Lilley, Signals of positive selection in genomes of palearctic Myotis-bats coexisting with a fungal pathogen, BMC Genomics 25:1, 828 (2024) Open Access Full Text
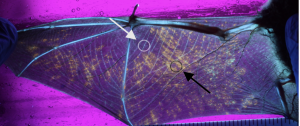
F Whiting-Fawcett, KA Field, SJ Puechmaille, AS Blomberg, TM Lilley, Heterothermy and antifungal responses in bats, Current Opinion in Microbiology 62, 61-67 (2021) Open Access Full Text
TM Lilley, IW Wilson, KA Field, DAM Reeder, ME Vodzak, GG Turner, A Kurta, AS Blomberg, S Hoff, CJ Herzog, BJ Sewall, S Paterson, Genome-Wide Changes in Genetic Diversity in a Population of Myotis lucifugus Affected by White-Nose Syndrome, G3: Genes, Genomes, Genetics 10 (6), 2007-2020 (2020) Open Access Full Text
TM Lilley, JM Prokkola, AS Blomberg, S Paterson, JS Johnson, GG Turner, T Bartonička, E Bachorec, DM Reeder, KA Field, Resistance is futile: RNA-sequencing reveals differing responses to bat fungal pathogen in Nearctic Myotis lucifugus and Palearctic Myotis myotis, Oecologia, 191(2), 295-309, doi:10.1007/s00442-019-04499-6 (2019) Open Access Full Text
K Hirose, AY Payumo, S Cutie, A Hoang, H Zhang, R Guyot, D Lunn, RB Bigley, H Yu, J Wang, M Smith, E Gillett, SE Muroy, T Schmid, E Wilson, KA Field, DM Reeder, M Maden, MM Yartsev, MJ Wolfgang, F Grützner, TS Scanlan, LI Szweda, R Buffenstein, G Hu, F Flamant, JE Olgin, GN Huang, Evidence for hormonal control of heart regenerative capacity during endothermy acquisition, Science 364 (6436), 184-188 doi:10.1126/science.aar2038 (2019)
KA Field, BJ Sewall, JM Prokkola, GG Turner, MF Gagnon, TM Lilley, JP White, JS Johnson, CL Hauer, DM Reeder, Effect of torpor on host transcriptomic responses to a fungal pathogen in hibernating bats, Molecular Ecology, doi:10.1111/mec.14827 (2018)
SM Reeder, JM Palmer, JM Prokkola, TM Lilley, DAM Reeder, KA Field, Pseudogymnoascus destructans transcriptome changes during white-nose syndrome infections, Virulence, doi:10.1080/21505594.2017.1342910 (2017) Open Access Full Text
MS Moore, KA Field, MJ Behr, GG Turner, ME Furze, DWF Stern, PR Allegra, SA Bouboulis, CD Musante, ME Vodzak, ME Biron, MB Meierhofer, WF Frick, JT Foster, DHowell, JA Kath, A Kurta, G Nordquist, JS Johnson, TM Lilley, BW Barrett, DM Reeder, Energy conserving thermoregulatory patterns and lower disease severity in a bat resistant to the impacts of white-nose syndrome, Journal of Comparative Physiology B, 1-12. doi:10.1007/s00360-017-1109-2 (2017) Full Text
TM Lilley, JM Prokkola, JS Johnson, EJ Rogers, S Gronsky, A Kurta, DM Reeder*, KA Field*, Immune responses in hibernating little brown myotis (Myotis lucifugus) with white-nose syndrome, Proceedings of the Royal Society B, 284(1848) 20162232. doi:10.1098/rspb.2016.2232 (2017) *Contributed equally, Full Text
TM Lilley, CA Wilson, RF Bernard, EV Willcox, EJ Vesterinen, QMR Webber, L Kurpiers, JM Prokkola, I Ejotre, A Kurta, KA Field, DM Reeder, AT Pulliainen, Molecular Detection of Candidatus Bartonella mayotimonensis in North American Bats, Vector-Borne and Zoonotic Diseases 17(4): 243-246. doi:10.1089/vbz.2016.2080 (2017) Full Text
TM Lilley, JS Johnson, L Ruokolainen, EJ Rogers, CA Wilson, SM Schell, KA Field*, DM Reeder*, White-nose syndrome survivors do not exhibit frequent arousals associated with Pseudogymnoascus destructans infection, Frontiers in Zoology 13(1) 1. doi:10.1186/s12983-016-0143-3 (2016) *Contributed equally. Open Access Full Text
KA Field, JS Johnson, TM Lilley, SM Reeder, EJ Rogers, MJ Behr, DM Reeder, The white-nose syndrome transcriptome: Activation of anti-fungal host responses in wing tissue of hibernating little brown myotis, PLoS Pathogens 11(10) e1005168. doi:10.1371/journal.ppat.1005168 (2015) Open Access Full Text
JS Johnson, DM Reeder, TM Lilley, GA Czirjak, CC Voigt, JW McMichael III, MB Meierhofer, CW Seery, SS Lumadue, AJ Altmann, MO Toro, KA Field, Antibodies to Pseudogymnoascus destructans are not sufficient for protection against white-nose syndrome, Ecology and Evolution 5(11): 2203–2214. doi:10.1002/ece3.1502 (2015) Open Access Full Text
JS Johnson, DM Reeder, JW McMichael III, MB Meierhofer, DWF Stern, SS Lumadue, LE Sigler, HD Winters, ME Vodzak, A Kurta, JA Kath, KA Field, Host, pathogen, and environmental characteristics predict white-nose syndrome mortality in captive little brown myotis (Myotis lucifugus), PLoS ONE 9(11): e112502. doi:10.1371/journal.pone.0112502 (2014) Open Access Full Text
Gaylo AE, Laux KS, Batzel EJ, Berg ME, Field KA. Delayed rejection of MHC class II-disparate skin allografts in mice treated with farnesyltransferase inhibitors. Transplant Immunology 20(3):163-70 (2009), Full Text
Field KA, Charoenthongtrakul S, Bishop JM, Refaeli Y. Farnesyl transferase inhibitors induce extended remissions in transgenic mice with mature B cell lymphomas. Molecular Cancer. 7:39 (2008). Open Access Full Text